The best way to prevent hydrate formation (and corrosion) is to keep the pipelines, tubing and equipment dry of liquid water. There are occasions, right or wrong, when the decision is made to operate a line or process containing liquid water. If this decision is made, and the process temperature is below the hydrate point, inhibition of this water is necessary.
Many materials may be added to water to depress both the hydrate and freezing temperatures. For many practical reasons, a thermodynamic hydrate inhibitor (THI) such as an alcohol or one of the glycols is injected, usually methanol, diethylene glycol (DEG) or monoethylene glycol (MEG). All may be recovered and recirculated, but the economics of methanol recovery may not be favorable in many cases. Hydrate prevention with methanol and or glycols can be quite expensive because of the high effective dosage required (10% to 60% of the water phase). Large concentrations of solvents aggravate potential scale problems by lowering the solubility of scaling salts in water and precipitating most known scale inhibitors. The total injection rate of inhibitor required is the amount/concentration of inhibitor in the liquid water phase for the desired hydrate temperature suppression, plus the amount of inhibitor that will distribute in the vapor and liquid hydrocarbon phases. Any inhibitor in the vapor phase or liquid hydrocarbon phase has little effect on hydrate formation conditions. Due to the accuracy limitations of the hydrate depression calculations and flow distribution in the process, it is recommended that the hydrate formation temperature with inhibition be chosen with a design factor below the coldest expected operating temperature of the system to ensure adequate inhibitor injection rates.
Determination of the amount and concentration of inhibitors and their distribution in different phases are very important for practical purposes and industrial applications. Therefore, to determine the required amount and concentration of these inhibitors, several thermodynamic models for hand and rigorous calculations have been developed and incorporated into computer software.
Low dosage inhibitors are relatively new and only recently reaching the “proven technology” stage in oil and gas processing. Although these systems move the hydrate formation line to the left, it is only temporary. In typical systems they will “delay” the formation of hydrates for about 12 hours. Low Dosage Hydrate Inhibitors (LDHIs) are two class of chemicals: Kinetic inhibitors (KHIs) and Anti-Agglomerants (AAs). A KHI can prevent hydrate formation but cannot dissolve an already formed hydrate. Current KHIs have a difficult time overcoming a subcooling temperature (ΔT) threshold of 15 °C (27 °F). AAs allow hydrates to form and maintain a stable dispersion of hydrate crystals in the hydrocarbon liquid. AAs form stable water in oil micro-emulsion. AAs adsorb onto the hydrate crystal lattice and disrupt further crystal growth but must have a liquid hydrocarbon phase and the maximum water oil ratio is about 40-50%.
Laboratory studies and field experiences indicate hydrate-inhibition synergy is gained through the combination of two or more THIs [1] or THI and LDHI [2]. This is termed a hybrid hydrate inhibition (HHI).
In this TOTM we will demonstrate the synergy effect of mixed THIs like NaCl and MEG solution. In the next TOTM, we will discuss the results of a successful application of combined methanol and a KHI solution for a well producing natural gas, condensate and water in the Gulf of Mexico (GOM).
Combined THIs (MEG + NaCl or MEG + KCl)
The produced water from natural gas reservoirs contains an electrolyte solution such as NaCl, KCl, and CaCl2. In order to estimate the hydrate formation temperature in the presence of mixed thermodynamic inhibitors, we propose to add up the depression temperature due to each individual inhibitor. The steps are summarized below:
- Using a conventional method described in reference [3], estimate the hydrate formation temperature in the presence of pure water, To.
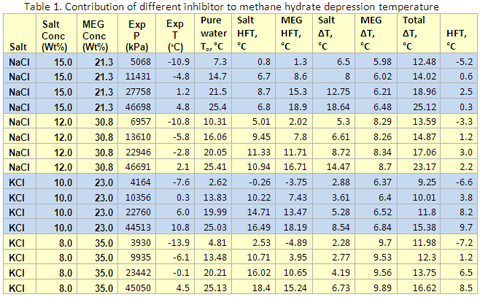
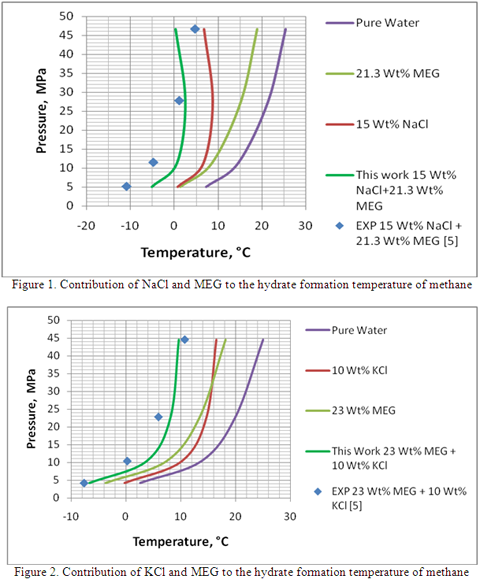
- Using a method similar to Javanmardi et al. [1], estimate the hydrate depression temperature due to the presence of salt solution, salt ΔT.
- Using a method similar to Hammerschmidt [4], estimate the hydrate depression temperature due to the presence of MEG solution, MEG ΔT.
- Add up Salt ΔT and MEG ΔT, Total ΔT.
- The hydrate formation temperature is calculated by subtracting total ΔT from To.
As an example, Table 1 presents the detail of calculation and the contribution of each inhibitor to the hydrate formation temperature for methane gas at different pressures and mixed inhibitor concentration. Comparison of the estimated hydrate formation temperature (last column of Table 1) with the experimental data (the fifth column) measured by Masoudi et al. [5] indicates a relatively good agreement. Figures 1 and 2 also present the contribution of each inhibitor to the hydrate formation temperature as described above for mixed solution of NaCl + MEG and KC l+ MEG, respectively.
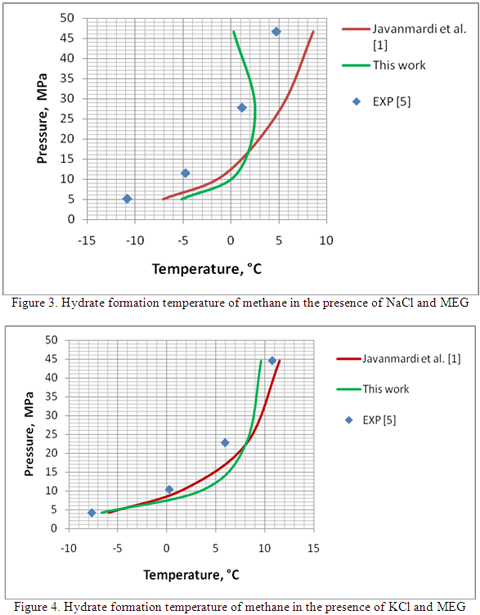
Table 2 presents a comparison between the accuracy of the proposed method with Javanmardi et al. method against the experimental data for methane gas in the presence of mixed inhibitors. Table 2 also indicates an average absolute temperature difference of 4.7 and 3.5 °C for the proposed method and Javanmardi et al. method, respectively.
In summary, a simple procedure is proposed for estimation of the hydrate formation temperature in the presence of mixed THIs such as MEG plus a salt solution. This procedure can be used for a mixture of glycol and electrolyte solutions. The procedure is relatively simple and its accuracy is good enough for facility calculations. For more accurate prediction of hydrate formation temperature in the presence of electrolytes, the readers should refer to the papers presented by Javanmardi et al. [1] and Masoudi et al. [5].
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing – Special) courses.
John M. Campbell Consulting (JMCC) offers consulting expertise on this subject and many others. For more information about the services JMCC provides, visit our website at www.jmcampbellconsulting.
By: Dr. Mahmood Moshfeghian
Reference:
- Javanmardi, J., Moshfeghian, M. and R. N. Maddox, “An Accurate Model for Prediction of Gas Hydrate Formation Conditions in Mixture of Aqueous Electrolyte Solutions and Alcohol,”Canadian J. of Chemical Engineering, 79, 367-373, (2001).
- Szymczak, S., Sanders, K., Pakulski, M., Higgins, T.; “Chemical Compromise: A Thermodynamic and Low-Dose Hydrate-Inhibitor Solution for Hydrate Control in the Gulf of Mexico,” SPE Projects, Facilities & Construction, (Dec 2006).
- Campbell, J. M., “Gas Conditioning and Processing”, Vol. 1, The Basic Principles, 8th Ed., Second Printing, J. M. Campbell and Company, Norman, Oklahoma, (2002).
- Hammerschmidt, E. G. “Formation of Gas Hydrate in Natural Gas Transmission Lines”, Ind. Eng. Chem., 26, 851-855, (1934).
- Masoudi, R., Tohidi, B., Anderson, R., Burgass, R., and Yang, J. “Experimental Measurement and Thermodynamic Modelling of Clathrate Hydrate Equilibria and Salt Solubility in Aqueous Ethylene Glycol and Electrolyte Solutions,” Fluid Phase Equilibria, 219, 157-163 (2004).


بسمه تعالی
با عرض سلام و خسته نباشید
خدمت با سعادت استاد گرانقدر
جناب آقای دکترمشفقیان
احتراما، اینجانب خدیجه رستمی دانشجوی کارشناسی ارشد مهندسی شیمی در دانشگاه سمنان می باشم که موضوع پایان نامه بنده،بررسی هم افزایی بازدارنده های هیدرات گازی است.
خواهشمندم در صورت امکان بنده را در این زمینه، با توجه به منبع دانش وسیع خوددر این زمینه یاری فرمایید
با نهایت سپاس گزاری
رستمی
Appreciating the dedication you put into your website and detailed
information you provide. It’s good to come across a
blog every once in a while that isn’t the same unwanted rehashed material.
Fantastic read! I’ve saved your site and I’m including your RSS feeds to my Google
account.
There’s definately a great deal to find out about this topic. I love all the points you’ve made.
I love looking through and I conceive this website got some really useful stuff on it! .
Can use this aproach for gas inyection well?
In Peru have a field where we inyect gas from a platform to other platform through 1 mille pipeline (inyection gas well, whp= 3000 psi) and everyday the compressors shut down for high pressure discharge because hydrates block pipeline. Actually we are pumping Methanol. Can we add salt?