The December 2012 Tip of the Month (TOTM) [1] discussed the hydrate phase behavior of sour natural gas mixtures. Specifically, it showed carbon dioxide inhibits the hydrate formation slightly while hydrogen sulfide enhances hydrate formation considerably. This tip will extend the previous study on the natural gas hydrate formation phase behavior. Specifically, it will study the impact of nitrogen on the formation of hydrate in a natural gas mixture.
The hydrate formation temperature of a gas depends on the system pressure and composition. There are several methods of calculating the hydrate formation conditions of natural gases [2-5]. References [2-3] present rigorous methods while [4-5] present the shortcut methods suitable for hand calculations. This study uses a rigorous method using the Soave-Redlich-Kwong (SRK) equation of state [6] in ProMax [7] software.
Table 1 presents the compositions of the gas mixture studied. Notice that in each case about 20 mole % of methane is replaced with the same amount of either nitrogen, carbon dioxide or hydrogen sulfide.
Table 1. Water-saturated compositions of gas mixtures studied
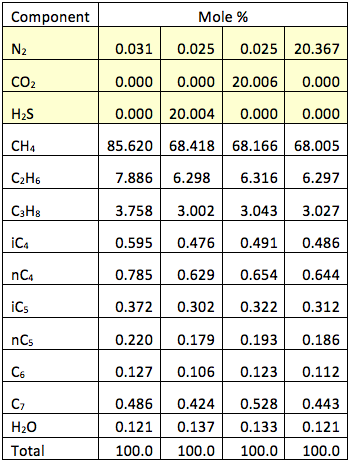
Figure 1 presents the calculated hydrate formation curve (broken curve) and the dew point portion of the phase envelope of a sweet natural gas (continuous curve). Figure 1 also presents the dew point and hydrate formation curves for the gas mixture containing 20 mole % nitrogen (N2).
Figure 1 indicates that the presences of 20 mole % N2 shifts the hydrate formation curves slightly to the left, depressing the hydrate formation temperature. Note that the points to the left and above the hydrate curves represent the hydrate formation region. From an operational point of view, this region should be avoided. This figure also indicates that the presence of N2 increases the cricondenbar and the two-phase (gas + liquid) region within the envelope expands.
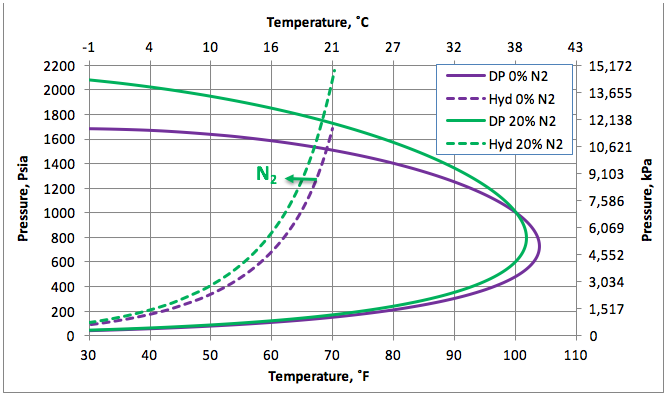
Figure 1. The impact of N2 on the hydrocarbon dew point and hydrate formation curves.
Figure 2 presents the calculated hydrate formation curves for a sweet gas (Continuous curve) with no N2, sour gases containing 20 mole % CO2 or H2S, and a sweet gas containing 20 mole % N2 (broken curves). This figure clearly indicates that the impact of N2 is much less than of H2S and slightly less than of CO2. Nitrogen and carbon dioxide depresses the hydrate formation condition slightly (shift the hydrate curves to the left) but H2S promotes hydrate formation considerably. As an example, at 1000 psia (6900 kPa), N2 reduces hydrate formation temperature for this sweet gas by about 4.5˚F (2.5˚C), CO2 reduces hydrate formation temperature by about 5.5˚F (3˚C) while, H2S increase the hydrate formation temperature by about 20˚F (11.1˚C).
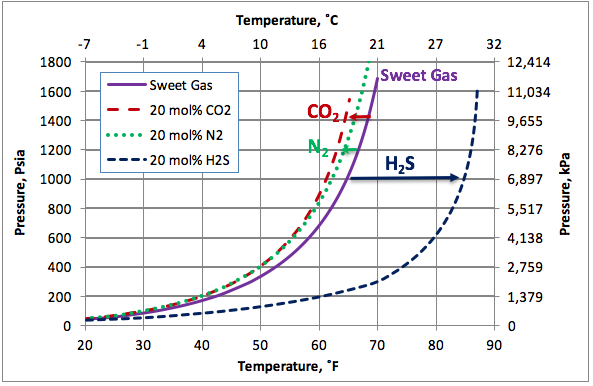
Figure 2. The impact of on non-hydrocarbons on the hydrocarbon hydrate formation curve.
Conclusions:
Katz and co-workers [8] developed a set of vapor-solid equilibrium constants (Kv-s) values for hydrate prediction. In the Katz method as described on page 161 of Chapter 6 of reference [6] “nitrogen is a hydrate former, and it is likely that some nitrogen may end up in the hydrate lattice in typical natural gas production systems. However, it is not a factor in determining hydrate formation conditions unless you are working with mixtures of nitrogen and methane which are sometimes found in coalbed methane production. In these cases the N2-CH4 mixture will have a lower hydrate formation temperature than pure methane. As a practical matter using Kv-s = (infinity) for nitrogen gives satisfactory results for typical natural gas mixtures”.
This study has showed that the presence of N2 and CO2 and H2S in natural gas has an opposite impact on the hydrate formation condition. While the impact of N2 and CO2 is small in the same direction, H2S has considerable impact on the hydrate formation condition in the opposite direction. For the same composition and condition studied, nitrogen and carbon dioxide slightly depresses hydrate formation (acts as hydrate inhibitor and shifts the hydrate curve to the left) while H2S shifts the hydrate curve to the right considerably, promoting hydrate formation conditions, and may cause severe operational problems.
To learn more about similar cases and how to minimize operational problems, we suggest attending our G4 (Gas Conditioning and Processing), G5 (Advanced Applications in Gas Processing), P81 (CO2 Surface Facilities), and PF4 (Oil Production and Processing Facilities), courses.
PetroSkills offers consulting expertise on this subject and many others. For more information about these services, visit our website at http://petroskills.com/consulting, or email us at consulting@PetroSkills.com.
By: Dr. Mahmood Moshfeghian
Reference:
- Moshfeghian, M. http://www.jmcampbell.com/tip-of-the-month/2012/12/sour-gas-hydrate-formation-phase-behavior/
- Parrish, W.R., and J.M. Prausnitz, “Dissociation pressures of gas hydrates formed by gas mixtures,” Ind. Eng. Chem. Proc. Dev. 11: 26, 1972.
- Holder, G. D., Gorbin, G. and Papadopoulo, K.D, “Thermodynamic and molecular properties of gas hydrates from mixtures containing methane. argon, and krypton,” Ind. Eng. Chem. Fund. 19(3): 282, 1980.
- Campbell, J.M., Gas Conditioning and Processing, Volume 1: The Basic Principles, 9th Edition, 2nd Printing, Editors Hubbard, R. and Snow–McGregor, K., Campbell Petroleum Series, Norman, Oklahoma, 2014.
- Gas Processors Suppliers Association; “ENGINEERING DATA BOOK” 13th Edition – FPS; Tulsa, Oklahoma, USA, 2012.
- Soave, Chem. Eng. Sci. 27, 1197-1203, 1972.
- ProMax 3.2, Bryan Research and Engineering, Inc, Bryan, Texas, 2015.
- Carson, D. B. and D. L. Katz, Trans. AIME, Vol. 146, p. 150, 1942.

[…] Moshfeghian, M., http://www.jmcampbell.com/tip-of-the-month/2016/01/what-is-the-impact-of-nitrogen-on-the-natural-gas… […]
Yes, I agree there many software for prediction of hydrate formation conditions and its inhibition calculations.
I do not even know the way I stopped up here, but I
assumed this put up used to be great. I don’t know who
you might be but definitely you are going to a well-known blogger in case
you aren’t already. Cheers!