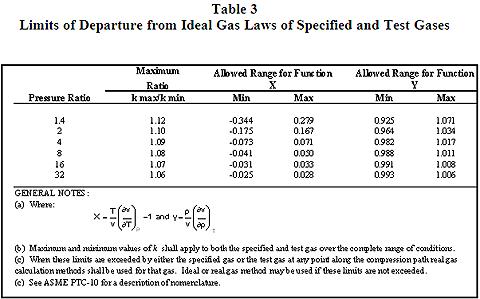
Introduction
Confusion sometimes results when reviewing published NPSHR curves. This is especially true when faced with trimming the impeller diameter to match changing operating conditions. A well known fact is that the head-flow relationship varies with the diameter. This can be accurately approximated by the affinity laws. However, what happens to the NPSHR-flow relationship when the diameter changes? This relationship is frequently over looked and can lead to pump cavitation. This Tip of the Month examines the relationship of NPSHR to the impeller diameter and clarifies other misconceptions regarding pump NPSHR curves.
Background
Pump performance may be shown for a single impeller or a range of impeller diameters. In the latter case the pump performance may be shown as multiple curves from the maximum to the minimum diameter, and may show several intermediate impeller sizes. In addition, the pump performance characteristics may show curves for NPSHR, efficiency and required power. The representation of the pump performance varies widely depending on many factors and can lead to design errors and possible confusion.
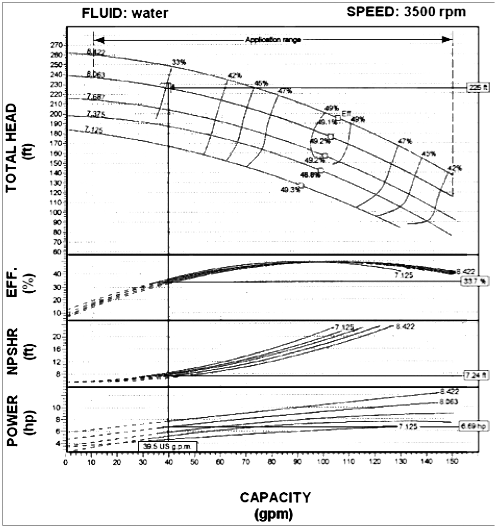
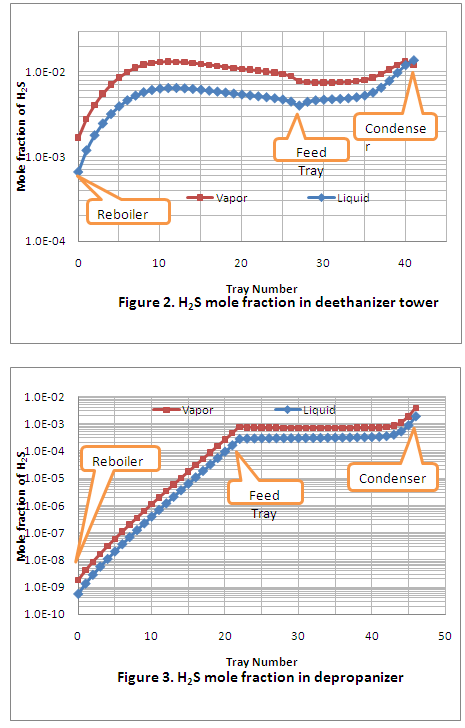
An example of a typical pump performance curve frequently seen in publications is shown in Figure 1. The pump flow rate is plotted on the horizontal axis, and the head and NPSHR curves, which are a function of flow rate, plotted on the vertical axles. Note that a single-line NPSHR curve starts at the no-flow condition and continually rises to the maximum flow rate. For several reasons that will be discussed later, this type of NPSHR curve is incorrect and can lead to design errors and possible cavitation problems.
Pump cavitation is a complex subject and the topic of many technical papers and books. However, it is widely accepted that this phenomenon begins at the pump inlet. It basically results from the increased velocity and reduced pressure as the fluid enters the impeller. If the fluid static pressure drops below the vapor pressure, gas bubbles form and later collapse as the fluid flows along the impeller vanes. These vapor bubbles can have a significant effect on the head produced by the pump.
It is important to note that fluid temperature also plays an important part in pump cavitation. Obviously, the fluid vapor pressure will vary with temperature. The fluid temperature will also vary with pump efficiency. Temperature rise due to pump efficiency is not significant in the high to mid-range flow rates, however, can be very significant at low flow rates. This is why pump NPSHR values are not given at low flow conditions.
Figure 1 – Pump Performance Curve for a Range of Impeller Diameters
Another important factor in pump cavitation is the fluid velocity. Fluid entering a pump will continually increase in velocity as it passes to the impeller eye. This increase in velocity causes a drop in the fluid static pressure and is analogous to lift on an airfoil. At high to mid-range flow rates the incoming fluid velocity and the impeller rotational velocity are compatible and contributes to stable flow through the pump. However at low flow rates the entering velocity is well below the rotational velocity and may cause the fluid to “recirculation” at the impeller inlet. Fluid recirculation is another form of pump cavitation. This is another reason why NPSHR is not given at low flow rates.
NPSHR Testing
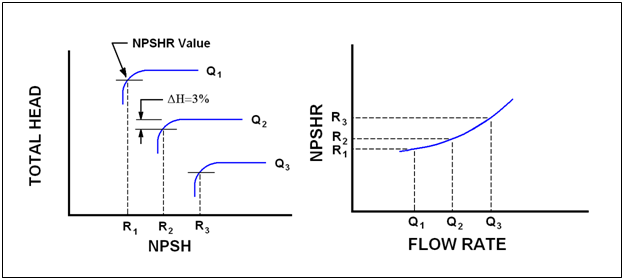
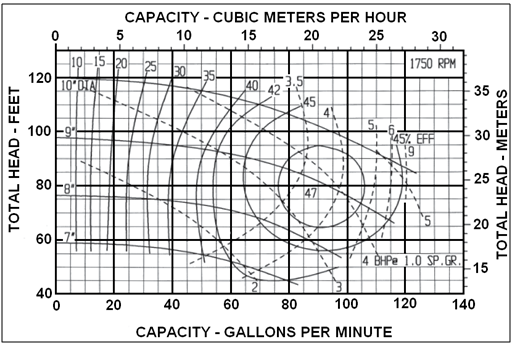
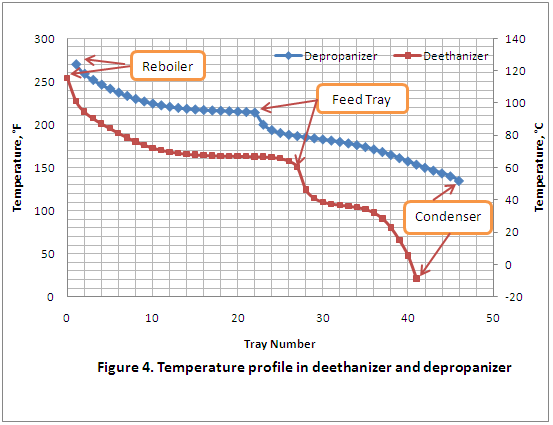
Understanding how NPSHR tests are conducted and how the impeller diameter influences the produced head will help eliminate confusion and possible errors. Pump manufacturers determine the characteristic shape of the NPSHR curve for each impeller through carefully controlled shop testing, hydraulic modeling and computer simulation. Hydraulic Institute Standard 1.6 gives strict guidelines for conducting shop testing and is used by most pump manufactures. Pumps are normally connected to closed-loop piping circuit where water flows from a suction tank (or sump) through the pump and then back to the tank. The discharge flow rate, temperature and pressure are carefully measured and controlled throughout the test. Basically the test is conducted at a fixed flow rate and speed while the suction pressure is reduced. By reducing the suction pressure a point is reached when the water begins to vaporize thus causing the pump to cavitate. The characteristic “cavitation” point is the flow rate that is exhibited by a small drop in head. The test is conducted again at another fixed flow rate and again the resulting suction pressure and flow rate value are recorded at the “cavitation” point. Once the series of tests are completed, a smooth line is drawn through the recorded data and plotted. Figure 2 illustrates a typical series of test results and the resulting NPSHR curve.
Figure 2 – NPSHR Test Curve
A pump cavitation point can be difficult to define. The formation of vapor bubbles is a gradual process, starting slowly and increasing with flow rate. The API-610 defines the cavitation point as a three percent drop in head. This is not to say that pump cavitation does not occur at smaller values, it is just difficult to accurately measure at smaller values. To obtain a single point it is necessary to run a pump for a period of time and allow the testing circuit to stabilize to the reducing suction pressure. Remember, vapor bubbles are forming and instruments need time to react to the fluid dynamics.
Impeller Diameter and Head Relationship
Larger pump impellers produce greater values of head for a given speed. This is because the head is proportional to the tip speed. The relationship of head to tip speed can be approximated by Equation 1.
(Eq. 1)
Tip velocity can also be related to impeller diameter and rotating speed by Equation 2.
(Eq. 2)
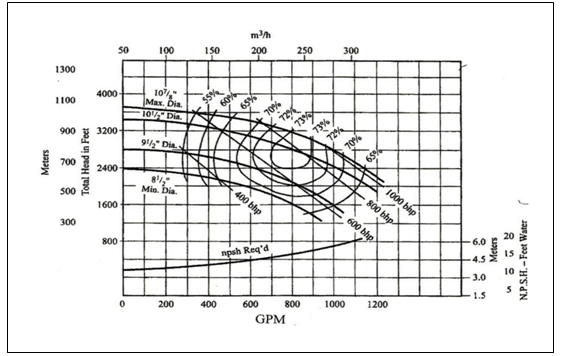
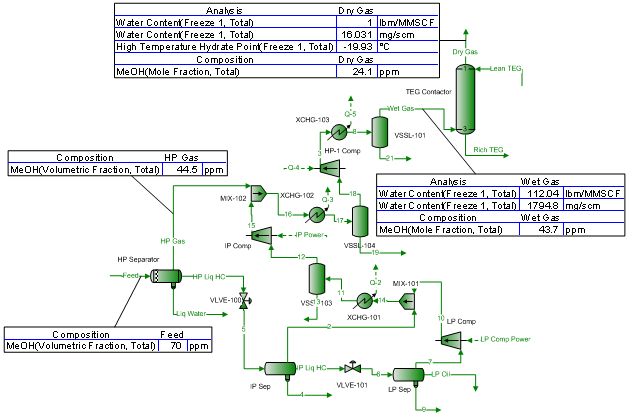
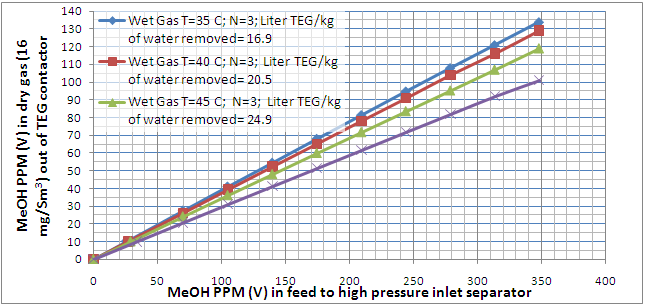
From Equations 1 and 2 it can be seen that changes in impeller diameter will have a direct effect on the pump head. For example, reducing the impeller diameter will lower the pump head by a factor of four. Since the cavitation point is identified by a three percent drop in pump head, it is logical that any change in impeller diameter will have a direct effect on the NPSHR value. For this reason, most pump manufacturers provide a single NPSHR curve for a given impeller diameter. Figures 3 and 4 are typical pump performance curves for a range of impeller diameters. Note that a separate NPSHR curve is given for each diameter.
Figure 3 – Typical Pump Performance Curve for a Range of Diameters
Figure 4 – Optional Pump Performance Curve for a Range of Diameters
Conclusions
The following conclusions can be reached from the previous discussion.
- Each impeller will have a characteristic NPSHR curve. It will depend on many design factors including the diameter.
- At a given flow rate, the NPSHR increases as the impeller diameter is reduced.
- The NPSHR is never tested at the shut-off point. The fluid temperature continually rises as the flow rates decreases. This prevents the system from stabilizing sufficiently to obtain accurate measurements.
- Pumps may cavitate at low flow rates due to recirculation of fluid at the impeller eye.
- The shape of the NPSHR curve is a U-shape. There is a slight rise in values as the flow is reduced and again at higher values. The NPSHR is lowest in the mid-range values.
By: Joe Honeywell
Legend
A Conversion constant = 720 ft/sec (600 m/s)
D Impeller diameter, inches (cm)
H Total pump head, ft (m)
g Gravitational constant, 32.17 ft/sec2 (9.81 m/s2)
n Rotational speed, rev/min
V Impeller tip velocity, ft/sec (m/s)
References
- American Petroleum Institute Standard 610, Centrifugal Pumps for Petroleum, Petrochemical and Natural Gas Industries, 10th Ed.
- Hydraulic Institute Standard 1.6, Centrifugal Pump Tests, 2000
- Terry Henshaw, Pumps and Systems, May 2009

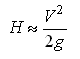
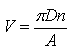

Dear Joe,
I am quit impressed to read your papers & hope you will assist us in future too.
Regards & Tanks,
Abhijit Raje
04 November, 2012
Dear Joe,
Your statement,
“A well known fact is head – flow relationship varies with diameter.
( AGREED ).
” This can be accurately (?) approximated by affinity laws
( I DO NOT AGREE )
All reputed manufacturers, factory test the Head-Capacity curves of their trimmed impellers and only then publish these curves for the respective diameters. More importantly,these trimmed tested curves are not parallel to each other, because the vane discharge angle increases with trimming and the curves are steeper. Will appreciate your comments.
Regards,
Arol Richard
There is no more.
I neededd to thnank you for this excellent read!!
I definitely lovd every biit of it. I have got you book-marked to check out new things yoou post…
Also viit my web bog stamped concrete
Fantastic sikte you have here but I was curious if you knew of any message boards
that cover the same topics discussed in this article? I’d really like to be a part of online communiity where I can get
feedback from other knowledgeable individuals that share the same interest.
If you have any suggestions, please let me know. Kudos!
my blog – stamped concreete nh – Richelle,
The Good water pump thks.
The Good water pump thks.
Dear Joe,
Excellent paper.
I have one issue where I cannot get a clear answer.
How does NPSHr vary for density i.e. the NPSHr curve is always established on water – does it change if the pump is used for a lower density fluid and if so how does the NPSHr curve change?
Aitken
I really like what you guys are up too. This sort of clever work and reporting! Keep up the awesome works guys I’ve added you guys to my blogroll.
Mittlerweile gibt es viele günstige Varianten der Tablette,
doch die Bezeichnung Aspirin ist Synonym für den gesamten Markt geblieben.
Dear Sir I have sarch sewage pump immpeller of how to calculate diameter& hight please answer me.
Dear Sir I have sarch sewage pump immpeller of how to calculate, formula diameter& hight please answer me.
Dort werden die Erkrankten rund um die Uhr betreut und erhalten bei Bedarf eine künstliche Ernährung
um den Körper zu stabilisieren.
Daher können Patienten mit zu hohen Blutdruckwerten aufgrund von Übergewicht die Ursache
selbst bekämpfen, indem sie abnehmen.
Nehme jetzt leider regelmäßig Medikamente und bin trotzdem dabei, meinen Lebensstil zu
ändern.
Die besten Tipps, die leckersten Rezepte und die spannendsten Erfolgsstories gibt es
auch per E-mail.
Dear Joe’
Thank you for information. I need design high flow rate and high pressure centifuruge pump. pressure will be 400 psi and flow rate will be verh high (i.e. 20.000 gpm). How can I determine the RPM and size of impeller?
Do you have any formula? I just need just draft numbers?
Regards..
Am stärksten sank der Blutdruck bei den Teilnehmern,
die vor dem Versuch einen oberen Wert von 129 mmHg hatten – nämlich um durchschnittlich
13,2 mmHg.
Dpa/Deutsche See Fischmanufaktur Lachs enthält viele gesunde Omega-3-Fettsäuren.
Und diese Rückkoppelung nach dem Motto „Meine Herde ist noch da könnte lebensrettend sein.
Schon sechs bis zehn Gramm dunkle Schokolade (Kakaoanteil mindestens 70 Prozent)
pro Tag können den Blutdruck senken.
Durch sportliche Betätigung vor allem im Ausdauerbereich sind positive Auswirkungen auf den Blutdruck nachgewiesen und nicht
zu verachten.
Je zwei Knoblauchzehen, Zwiebeln und Zitronen mit
der Schale schneiden und in einem Liter Wasser acht Minuten kochen lassen.
Medikamente Wer mit Medikamenten den Blutdruck senken muss, sollte sie genau nach Verordnung einnehmen, nie auf eigene Faust anders dosieren.
Eine gesunde Ernährung sollte daher an die persönlichen Wünsche und Bedürfnisse angepasst werden.
Hallo, Ihr Blog Thema zeigen sich nicht richtig auf dem Web-Browser NecroPedoSadoMaso 42 von SEWER… Es kommt wahrscheinlich aus dem WordPress-Theme oder Plugins. https://mmodeutsch.wordpress.com/2016/06/07/untoter-paladin-die-besten-world-of-warcraft-klasse/
Can you answer to my query “pump with higher NPSHr is better or with lower NPSHr particularly in case of higher head pumping
fantastic intel you got here how anyone’s first impressions on mine post
in respect to click me
great intel we have at this time what you’re comments on mine web post relative to bowling king online hack
Hey nice post. I hope it’s ok that I shared it on my Twitter, if not,
no problem just let me know and I’ll remove it. Either way keep up the good work.
Hi,I read your new stuff named “Why NPSHR Changes With Impeller Diameter? | Campbell Tip of the Month” on a regular basis.Your humoristic style is awesome, keep doing what you’re doing! And you can look our website about free anonymous proxies.
It’s going to be end of mine day, except before finish I am reading this fantastic paragraph to increase my
know-how.
Tremendous things here. I’m very satisfied to look your post.
Thanks a lot and I’m looking forward to contact you.
Willl youu kindly drop me a mail?
Hello, I think your blog might be having browser compatibility issues.
When I look at your blog in Opera, it looks fine but when opening in Internet Explorer, it has some overlapping.
I just wanted to give you a quick heads up! Other then that, fanntastic blog!