The best way to prevent hydrate formation (and corrosion) is to keep the pipelines, tubing and equipment dry of liquid water. There are occasions, rightly or wrongly, when the decision is made to operate a line or process containing liquid water. If this decision is made, and the process temperature is below the hydrate point, inhibition of this water is necessary. Many materials may be added to water to depress both the hydrate and freezing temperatures. For many practical reasons, an alcohol or one of the glycols is injected as an inhibitor, usually methanol, diethylene glycol (DEG) or ethylene glycol (EG). All may be recovered and recirculated, but the economics of methanol recovery may not be favorable in many cases. Total injection rate is that needed to provide the necessary inhibitor concentration in the liquid water plus that inhibitor which enters the vapor and hydrocarbon liquid phases. Any inhibitor in the vapor phase or liquid hydrocarbon phase has little effect on hydrate formation conditions. Determination of the amount and concentration of inhibitors and their distribution in different phases are very important for practical purposes and industrial applications. Therefore, in order to determine the required amount and concentration of these inhibitors, several thermodynamic models for hand and rigorous calculations have been developed and incorporated into the computer software.
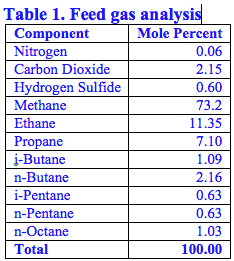
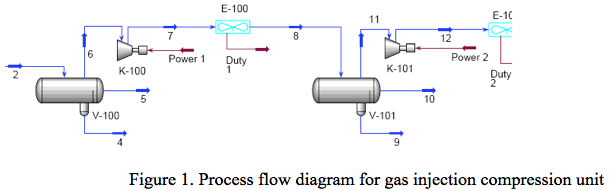
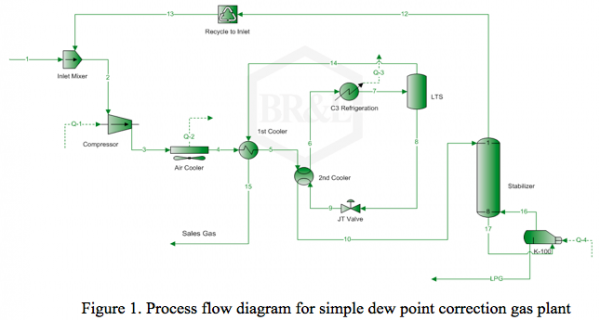
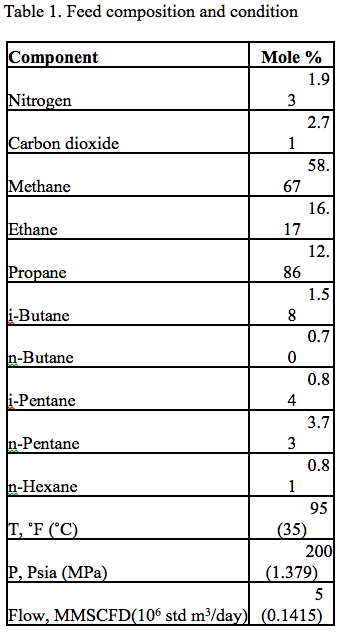
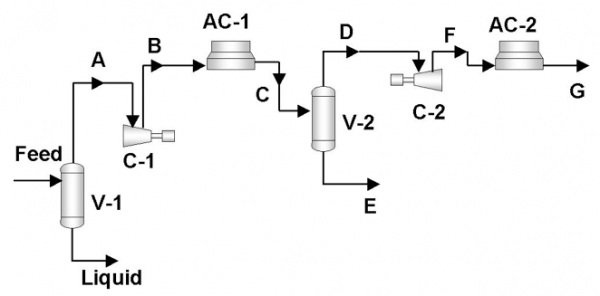
In this Tip of the Month, we will demonstrate the effect of total inhibitor circulation rate on hydrate depression in a sales gas dew point correction plant. Let’s consider the process flow diagram shown in Figure 1. The feed composition and conditions are shown in Table 1. The wet gas analysis was used in this study.
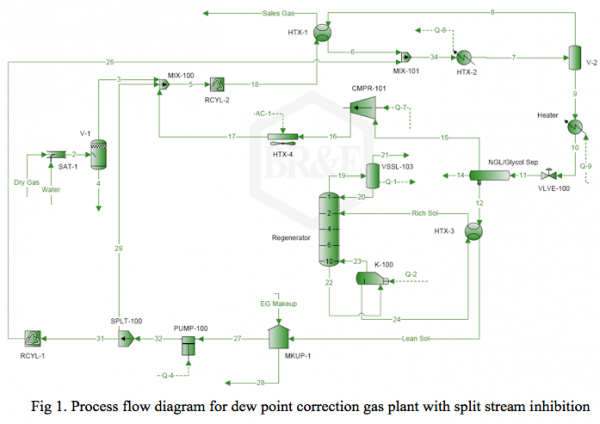
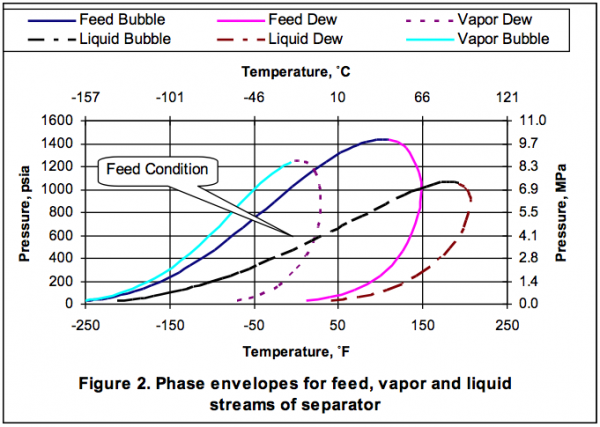
It is desired to process this feed gas to produce a sales gas with a dew point of -5˚F (-20.6˚C) at 990 psia (6.826 MPa) The feed gas is mixed with recycle gas from the NGL/Glycol separator, compressed and cooled to 120˚F (48.9˚C) and 1000 psia (6.895 MPa), and EG lean solution and then cooled in the HTX-1, and finally in the HTX-2 to -5˚F (-20.6˚C) before entering the separator at 990 psia (6.826 MPa). For the sake of simplicity, the refrigeration cycle for HTX-2 system is not shown in the above process diagram. Figure 2 represents the feed phase envelope, its hydrate curve, sales gas envelope and the cooling path. As seen in this figure, the gas temperature drops below the hydrate formation temperature of about 65˚F (18.3˚C) in the HTX-1 and to -5˚F (-20.6˚C) in HTX-2. Therefore; to prevent the hydrate formation in the HTX-1 and HTX-2, it is decided to inject an 80 weight percent lean EG solution to the gas stream. The concentration of rich inhibitor solution can be calculated by the shortcut method of Hammerschmidt [1] as described in Chapter 6, Volume 1 of Gas Conditioning and Processing [2] or using rigorous thermodynamic models. The required circulation rate is then determined by material balance and phase equilibrium calculations.
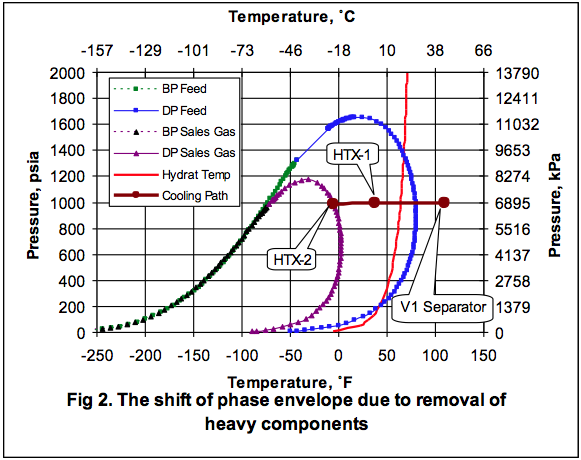
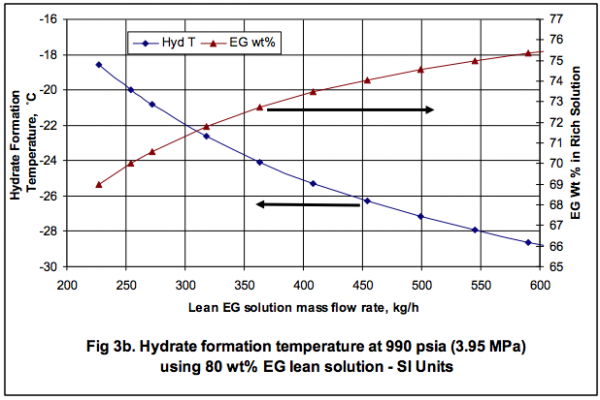
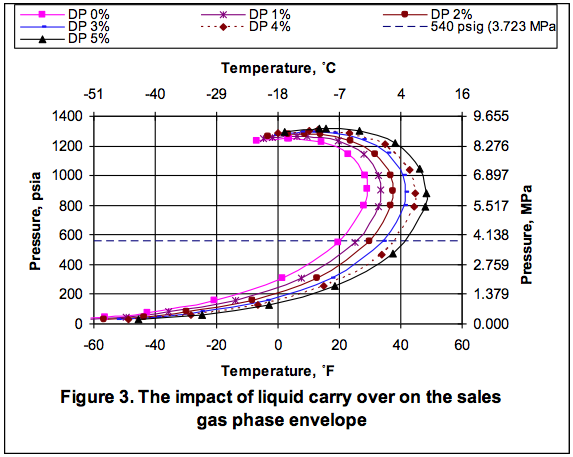
In order to show the effect of EG solution circulation rate on the depression of the hydrate formation temperature, the whole process shown in Figure 1. Figure 3 shows how the hydrate formation temperature in HTX-2 drops with increasing total inhibitor circulation rate. The corresponding calculated weight percent of EG in rich solution is also shown as a function of circulation rate. The concentration of EG in the rich inhibitor solution increases with the increase in the inhibitor solution circulation rate. Figure 3 indicates that for a 10˚F (5.6˚C) depression, or the hydrate formation temperature of -15˚F (-26.1˚C), a circulation rate of 975 lbm/hr (442 kg/h) is required. At this circulation rate the corresponding weight percent of EG in rich solution drops to 74.
To learn more about similar cases and how to find the optimum inhibitor circulation rate and concentration for prevention of hydrate formation, we suggest attending our G4 and G5 courses.
Dr. Mahmood Moshfeghian
References:
- Hammerschmidt, E.G., “Formation of gas hydrates in natural gas transmission lines,” Ind. & Eng. Chem., Vol. 26, p. 851, 1934
- Campbell, J. M., “Gas Conditioning and Processing, Vol. 1, the Equipment Modules, 8th Ed., J. M. Campbell and Company, Norman, Oklahoma, 2001
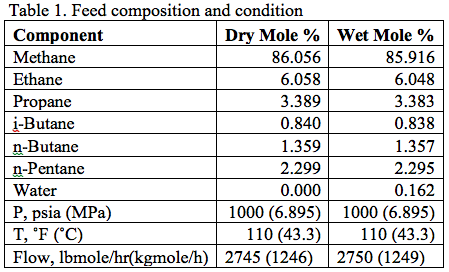

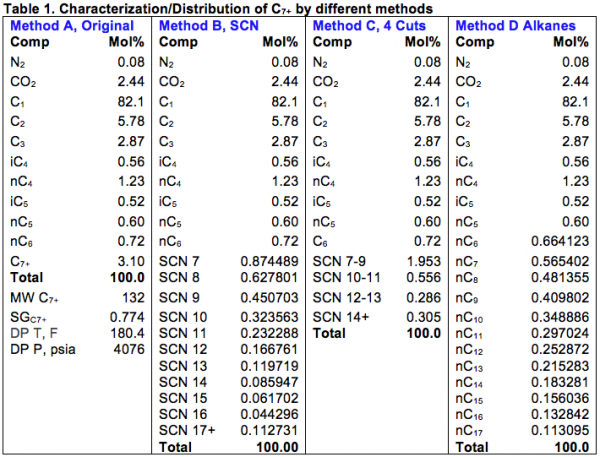
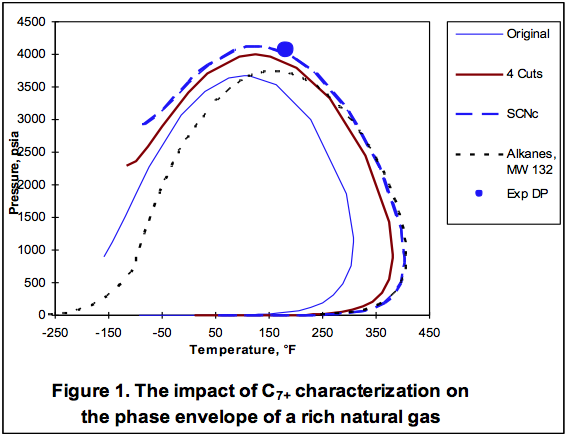
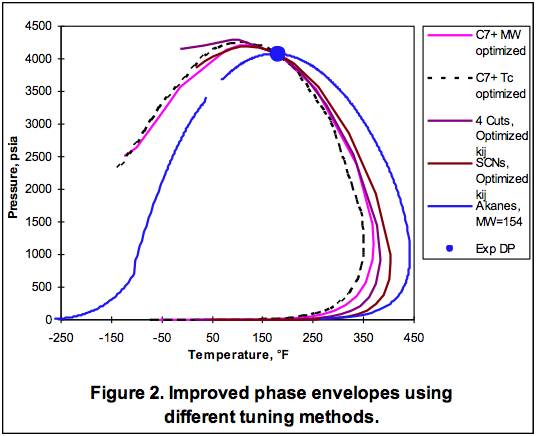
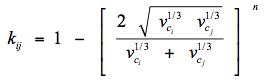
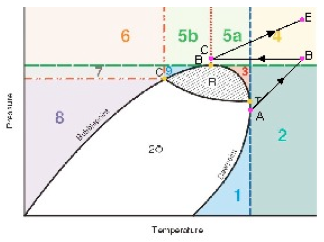
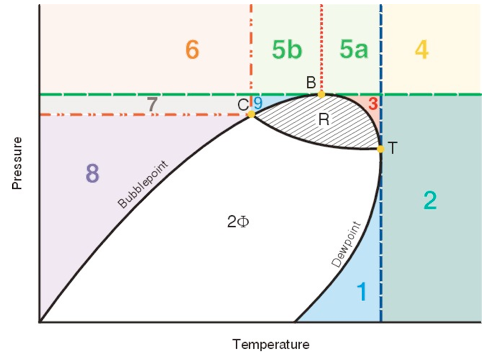
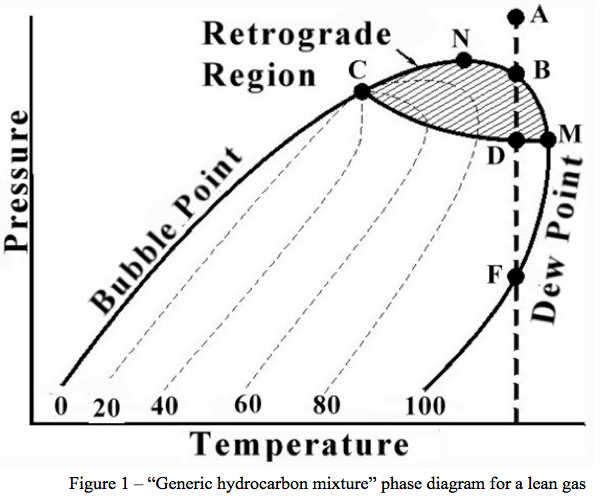 The area to the right of the Dew Point line is the super-heated gas region. Between these two lines the mixture is two-phase. Other areas of interest are the retrograde region and the supercritical region. Each of these regions provides advantages and disadvantages for operations.
The area to the right of the Dew Point line is the super-heated gas region. Between these two lines the mixture is two-phase. Other areas of interest are the retrograde region and the supercritical region. Each of these regions provides advantages and disadvantages for operations.
sir, in a compressed natural gas @ 3500 psig @ 15 DEG C (dense phase), HYSYS predicts hydrate temp.@ 25 deg C. is it possible to use glycols to inhibit hydrate formation?. Since in dense phase a distinct aqueous phase does not exist,is Hammerschmitt equation valid? has it been used for dense phase hydrate inhibition by use of THI. if not, what else is the remedy?
your kind views are solicited
sarvjeetsharma@hotmail.com
Does anyone know who can supply MEG injection Nozzles. We need to spray Meg liquid prior to a natural gas cooler to prevent hydrate formation. Who can provide MEG nozzles to assure complete coverage of inlet heat exchanger tubes.